Abstract
Introduction: Trial design and modern inclusion/exclusion criteria have impacted the patients enrolling in frontline trials for DLBCL. Our previous analysis from the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (Khurana et al. JCO 2021) identified that up to 24% of patients treated with standard immunochemotherapy (IC) were excluded based on 5 lab-based criteria alone. Additionally, ineligible patients had worse clinical outcomes and increased deaths related to lymphoma progression suggesting the potential exclusion of patients who could have benefited most from novel therapies being evaluated in these trials. We sought to evaluate the impact of laboratory eligibility criteria from recent first-line DLBCL trials in patients from the Lymphoma Epidemiology Outcomes (LEO) Cohort shown to have demographics similar to the US SEER population (Flowers et al. ASH 2018) and evaluated the impact of trial eligibility criteria by race/ethnicity.
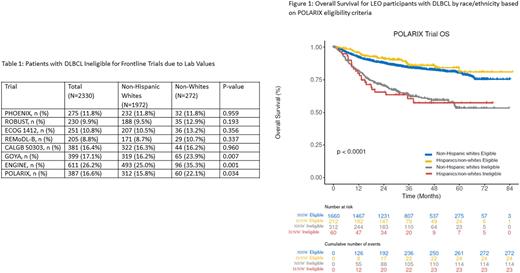
Methods: Newly diagnosed patients with DLBCL were enrolled from 2015-2020 into the LEO Cohort from 8 large academic centers across the US. Clinical data, including baseline laboratory values, were abstracted from the medical record using a standard protocol. Patients were prospectively followed for outcomes. All patients for this analysis received anthracycline plus CD-20 antibody-based IC to standardize treatment. Organ function parameters were identified from the exclusion criteria for hemoglobin, absolute neutrophil count, platelet count, creatinine, and bilirubin, as reported in recent frontline trials (Table 1). Associations between trial eligibility and event-free (EFS) and overall survival (OS) were evaluated using Kaplan Meier curves. The POLARIX trial was used as an example here for outcomes as it is the most recently published frontline DLBCL trial showing a benefit in clinical outcomes over the standard of care R-CHOP.
Results: A total of 2510 DLBCL patients received frontline IC. Of these 2330 had 3 or more of the 5 lab-based values available in LEO and comprised the analysis set. The group included 1972 non-Hispanic whites (NHW, 85%) and 272 Hispanic and/or non-whites (H/NW, 12%) participants with the rest unknown race/ethnicity. Compared to NHW, H/NWs were younger at diagnosis (median 54 vs. 64 years, p <0.001) but were comparable for elevated LDH (56% vs. 63%), B-symptoms (32% vs. 36%), bone marrow involvement (16% vs 18%), extranodal disease (26% vs 32%) and high-risk IPI (38% vs. 37%). Hemoglobin was significantly lower (mean + SD) in the H/NW (11.7±2.3) vs. NHW (12.5±2.2, p<0.001), while other labs values were similar between the two groups. Treatment regimens varied between the 2 groups with more NHW receiving R-CHOP (53% vs.38%, p <0.001), and more H/NWs receiving R-EPOCH (38% vs. 25%, p <0.001).
Within the LEO cohort 9-26% of patients would have been excluded from recent frontline DLBCL clinical trials based on the 5 lab-based criteria alone (Table 1). Of these, ENGINE (26%), POLARIX (17%), and GOYA (17%) trials were the most restrictive. Trials with a higher cut-off value of hemoglobin (9gm/dL or more) had a larger impact on H/NW population's ineligibility. OS was significantly inferior for patients ineligible for all trials listed in table 1 based on lab criteria for both H/NW and NHW patients [POLARIX as an example - Figure 1]. Associations between ineligibility and poor outcomes were consistent across trial criteria and endpoints (OS and EFS).
Conclusions: This study confirms our previously reported association between trial lab-based eligibility criteria and outcomes in newly diagnosed DLBCL in a larger, contemporary, diverse cohort and showed some criteria disproportionately limited eligibility for H/NW patients. Further studies to evaluate the impact of each lab-based criteria on eligibility in different racial/ethnic populations are required. Future trial designs need to tailor eligibility criteria to be more inclusive of the H/NW population.
Disclosures
Nastoupil:Genentech/Roche, MEI, Takeda: Other: DSMC; ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. Reagan:Seagen: Research Funding; Genentech: Research Funding; Kite, a Gilead Company: Consultancy; Caribou Biosciences: Consultancy. Farooq:MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Honoraria; Caribou pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Checkmate Pharma: Research Funding. Romancik:AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Lossos:NCI: Research Funding; Adaptive: Honoraria; LRF: Membership on an entity's Board of Directors or advisory committees. Kahl:AstraZeneca: Consultancy, Research Funding; ADT Therapeutics: Consultancy; Roche: Consultancy; Genentech: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; MEI: Consultancy; AcertaPharma: Consultancy; Pharmacyclics: Consultancy; Celgene/BMS: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Kite: Consultancy; Janssen: Consultancy; Incyte: Consultancy; Hutchmed: Consultancy, Research Funding; TG Therapeutics: Consultancy; Genmab: Consultancy; Seattle Genetics: Consultancy; Research To Practice: Speakers Bureau. Martin:ADCT: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; BMS: Consultancy; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Regeneron: Consultancy; Takeda: Consultancy. Witzig:Karyopharm: Other: Clinical Trail Support; Curio Science: Honoraria; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Other: Clinical Trail Support. Cerhan:BMS/Celgene: Research Funding; Genentech: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; NanoString: Research Funding; Protagonist: Membership on an entity's Board of Directors or advisory committees. Flowers:Denovo Biopharma: Consultancy; Genentech/Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Cellectis: Research Funding; Amgen: Research Funding; Allogene: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; NPower: Current holder of stock options in a privately-held company; 4D: Research Funding; SeaGen: Consultancy; Pharmacyclics/Janssen: Consultancy; Karyopharm: Consultancy; Genmab: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Spectrum: Consultancy; Gilead: Consultancy, Research Funding; EMD: Research Funding; Guardant: Research Funding; Iovance: Research Funding; Janssen Pharmaceutical: Research Funding; Kite: Research Funding; Morphosys: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; Burroughs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; BeiGene: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding. Maurer:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Roche/Genentech: Research Funding. Nowakowski:Bantam Pharmaceutical: Consultancy; Blueprint Medicines Corporation: Consultancy; Celgene Corporation/Bristol Myers Squibb: Consultancy, Research Funding; Curis, Inc.: Consultancy; Daiichi Sankyo Inc: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Genentech, Inc: Consultancy, Research Funding; Incyte: Consultancy; Karyopharm: Consultancy; Kite Pharma Inc.: Consultancy; Kymera Therapeutics: Consultancy; MorphoSys US Inc: Consultancy; NanoString: Research Funding; Ryvu Therapeutics: Consultancy; Selvita: Consultancy; TG Therapeutics: Consultancy; Zai Lab: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.